[Original Supplier] Regenovue Sub-Q - HA Dermal Filler
HA dermal filler without lido. CE Approved. Made in Korea. Brought to you by the original manufacturer - NeoGenesis Co., Ltd.
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Regenovue
- Payment Terms
- Others,T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- hyaluronic acid filler, nose filler, lip filler, ce filler

NeoGenesis Co., Ltd.
- Membership
- VIP
- Recent Visit
- Nov 25, 2024
- Country / Year Established
-
 South Korea
/
2012
South Korea
/
2012
- Business type
- Distributor
- Verified Certificate
-
9



| Product name | [Original Supplier] Regenovue Sub-Q - HA Dermal Filler | Certification | - |
|---|---|---|---|
| Category |
Other Personal Care
Beauty Equipment Medical Consumables Other Beauty Equipment Other Medical Consumables |
Ingredients | CROSSLINKED HYALURONIC ACID |
| Keyword | hyaluronic acid filler , nose filler , lip filler , ce filler | Unit Size | 19.5 * 3.0 * 7.2 cm |
| Brand name | Regenovue | Unit Weigh | 51 g |
| origin | South Korea | Stock | 0 |
| Supply type | Available | HS code | - |
Product Information

Regenovue Sub-Q
Korean HA Dermal Filler Without Lido (Lido Free)
- Cross-linked High Quality Hyaluronic Acid Dermal Filler
- Suitable for Patients Allergic to Lido
- 1ml x 1 Syringe per Box
- CE Certified
.
.
.
< Application Areas of Regenovue Sub-Q >
- Deep Wrinkles:
Nasolabial lines
- Facial Augmentation:
Forehead
Zygoma
Chin
Lips prosthesis
Nose ridge
.
.
.
< Product Strengths >
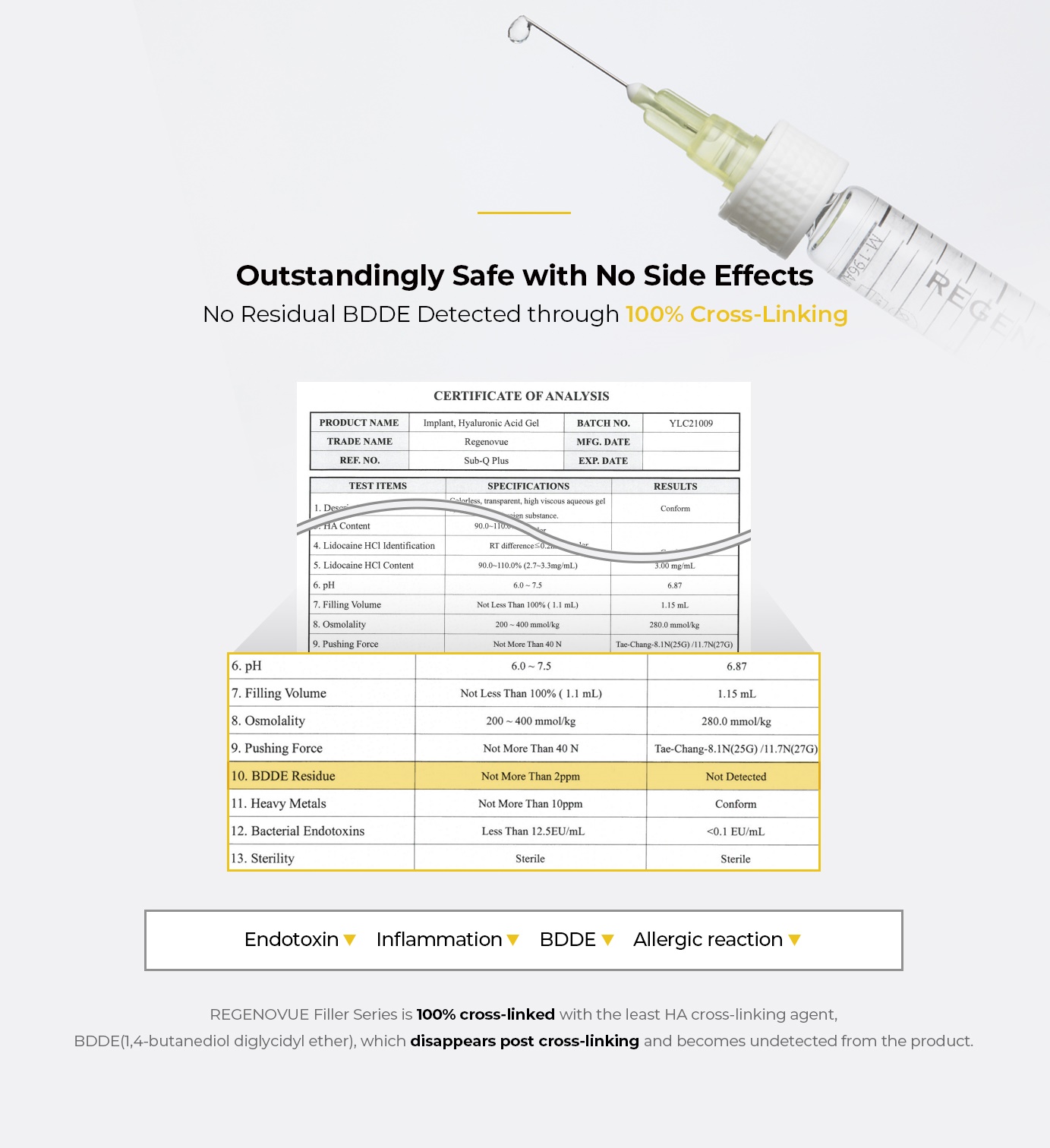
- 100% cross-linked with BDDE for no side effects
- Natural volumizing & precise contouring from unique monophasic cross-linking technique
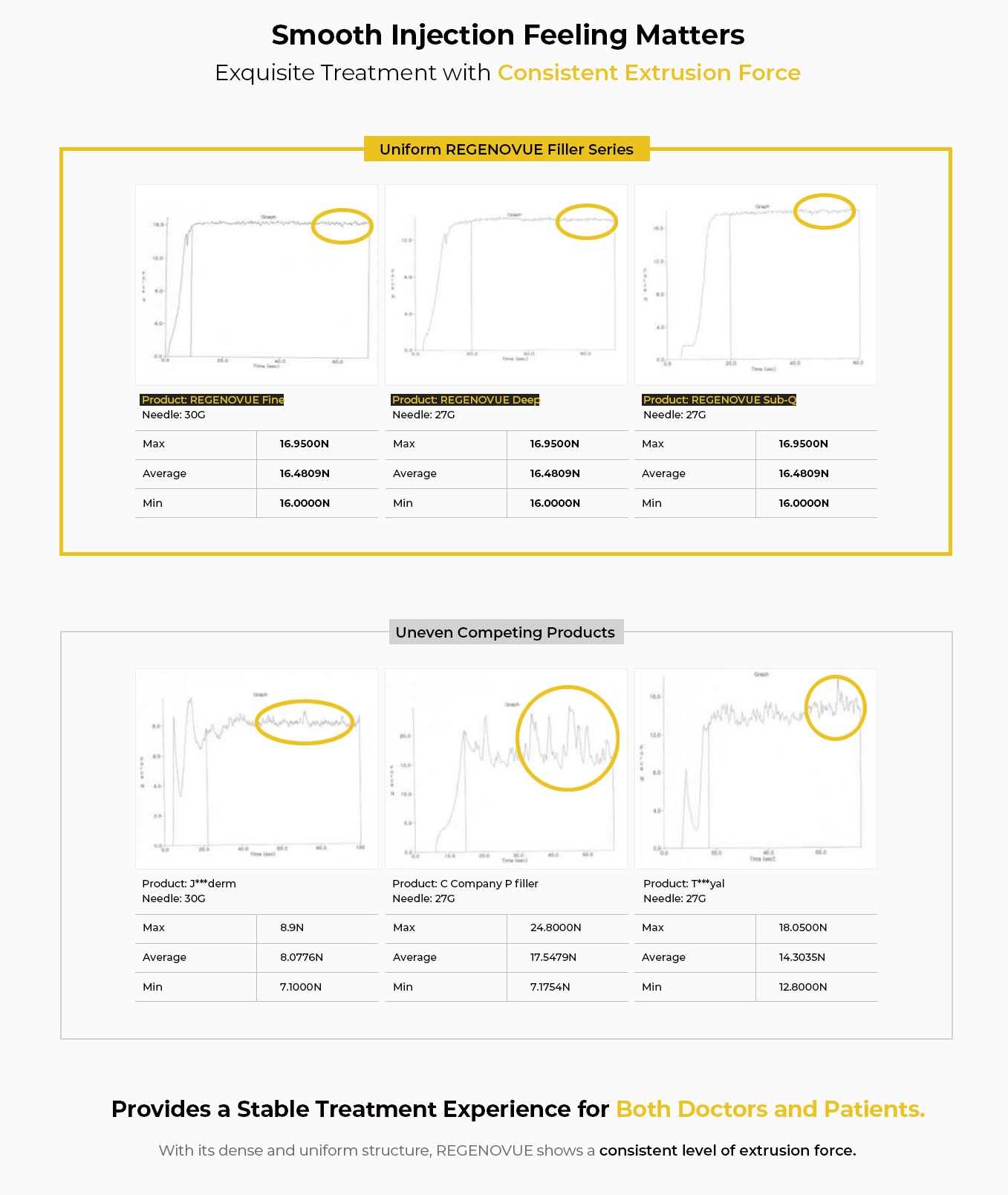
- Consistent injecting pressure for doctors enabling exquisite treatment
- Smooth and relatively less painful injection
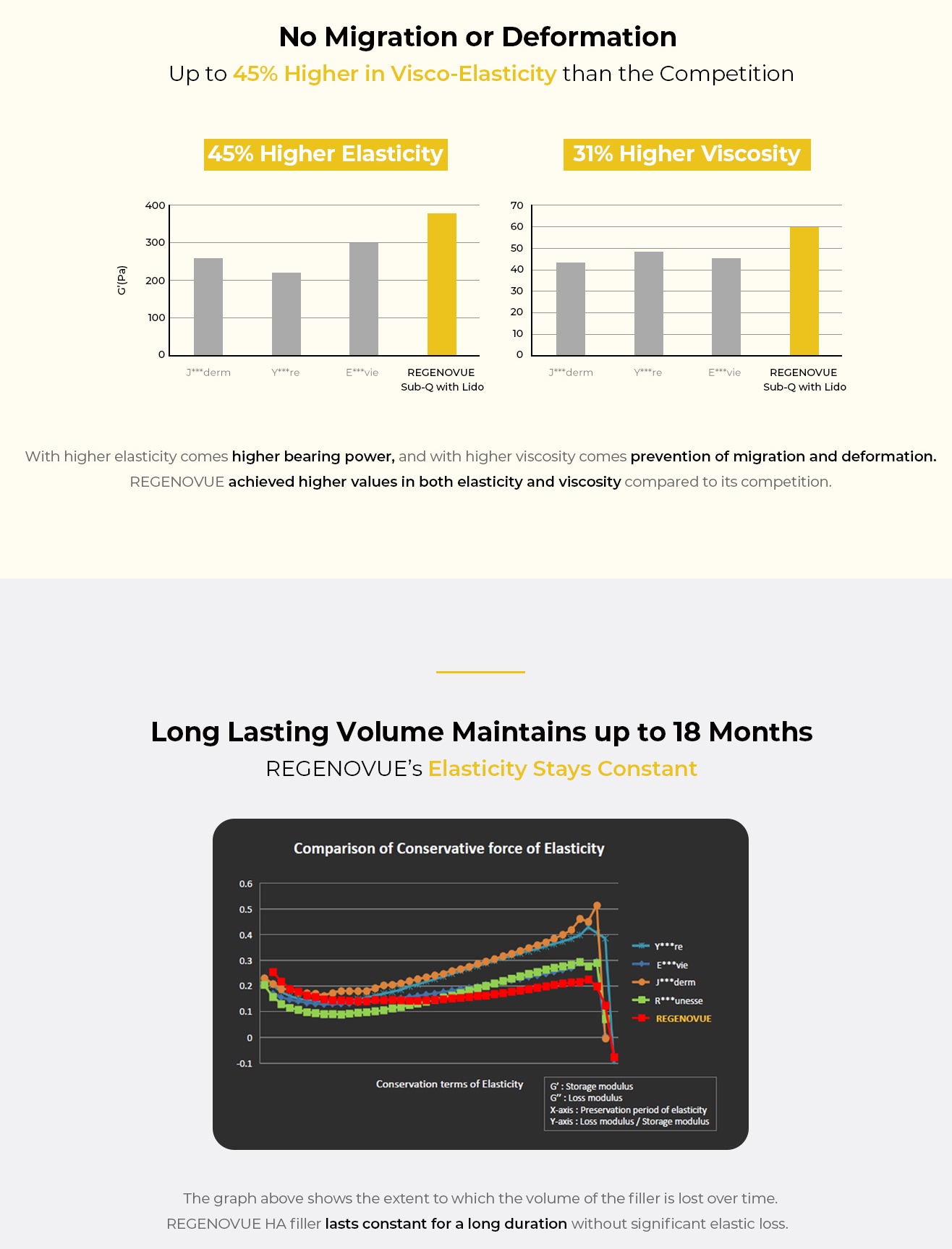
- No migration or deformation with up to 45% higher visco-elasticity than competition
- Long-lasting volume which lasts up to 18 months
- Pure non-animal based hyaluronic acid, providing safety from immune response and infections
- High viscosity
- High hydrophilicity
- Competitive market price
.
.
.
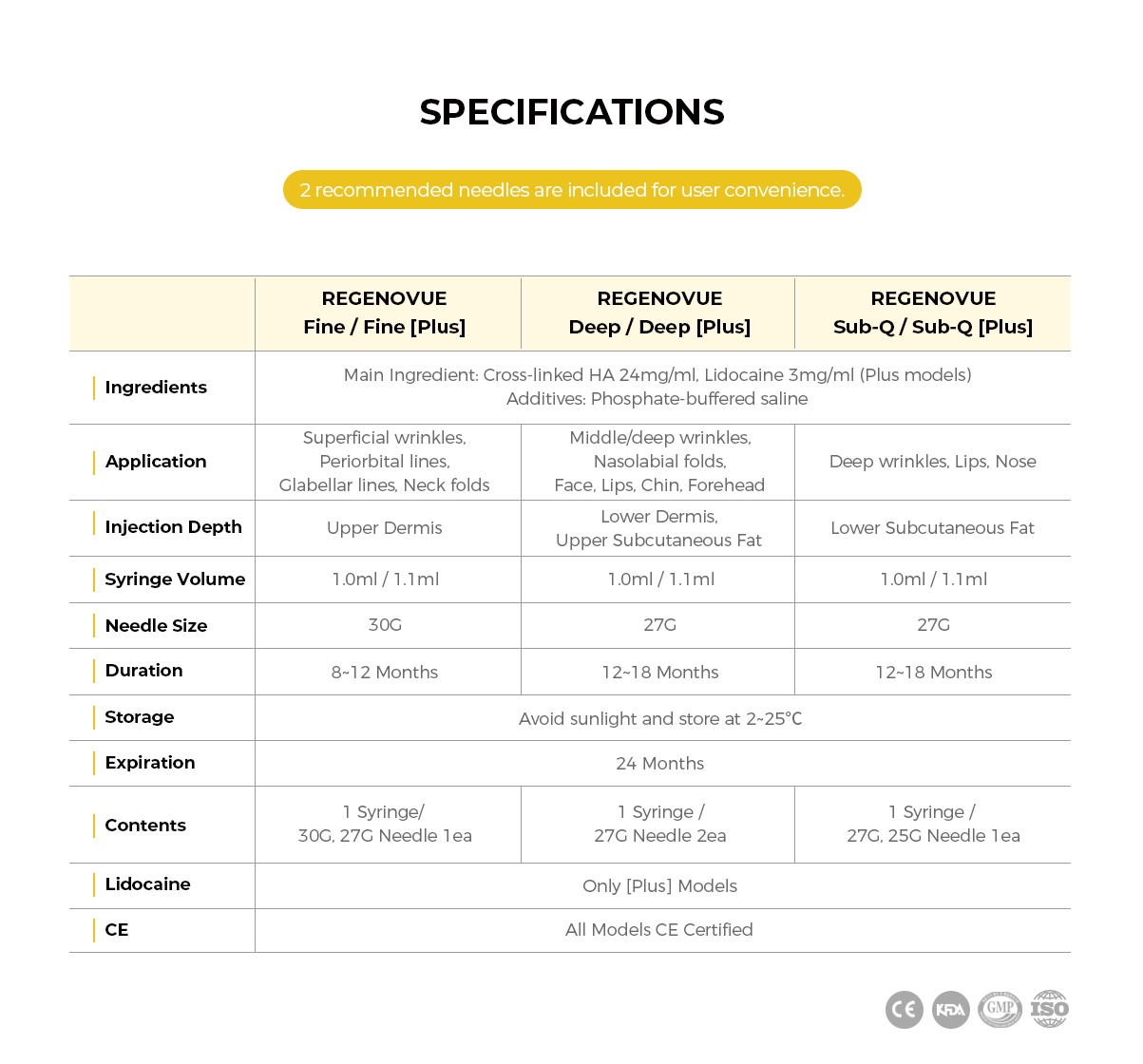
< Product Specifications >
- Ingredients: Cross-linked HA 24mg/ml (Main Ingredient), Phosphate-buffered Saline (Additive)
- Injection Depth: Lower Subcutaneous Fat
- Syringe Volume: 1ml x 1 per box
- Needle Size: 27G
- Duration: 8-12 Months
- Storage: Store at 2-25°C away from direct sunlight
- Expiration: 24 Months
- Contents: 1 Syringe & 27G Needle & 25G Needle
- Certifications: ISO 13485, GMP, KFDA, CE
- Country of Manufacture: South Korea
.
.
.
< Other Available Models >
- Regenovue Sub-Q Plus: Same model with lido for deep wrinkle treatment & facial augmentation
- Regenovue Deep / Deep Plus: For middle-deep wrinkle treatment
- Regenovue Fine / Fine Plus: For superficial wrinkle treatment
.
.
.
Regenovue Video Link: https://youtu.be/L42ENPHj2nc
Information also available on our website: http://www.neogenesis.co.kr/eng/item.php?it_id=1646384885
.
.
.
Contact us now for a sample order!
(Website) http://www.neogenesis.co.kr
(YouTube) https://www.youtube.com/@NeogenesisInfo/videos
(Facebook) http://www.facebook.com/neogenesis.coltd
(Twitter) twitter.com/neogenesiscoltd
(Tel) +82-2-583-1348
(FAX) +82-2-2038-0848
(Address) #302, E&C Dream Tower, 146, Seonyu-ro,
Yeongdeungpo-gu, Seoul, South Korea (07255)
.
.
.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,T/T | Shipping time | Negotiable |

NeoGenesis Co., Ltd.
- Country / Year Established
-
 South Korea
/
2012
South Korea
/
2012
- Membership
- VIP
- Recent Visit
- Nov 25, 2024
- Business type
- Distributor
-
9



- President
- Yongpil Kim
- Address
- #302 E&C Dream Tower, 146 Seonyu-ro, Yeongdeungpo-gu, Seoul, Korea
- Product Category
- Makeup,Medical Devices
- Year Established
- 2012
- Company introduction
-
As an enterprise specializing in autologous cell therapy, NeoGenesis develops and exports medical devices to the global market.
-
Since our foundation in 2012, we at NeoGenesis have strived to research and develop innovative medical devices. With expertise in the autologous cell therapy field, we provide our medical devices to not only the domestic market, but also to the global market including the Americas, Europe, Asia, and Africa.
-
We have made efforts to supply knowledge of modern cell therapy through participation in seminars, exhibitions, and training around the world, and now contribute to the global beauty industry as well.
-
NeoGenesis will continue to provide safety and quality guaranteed medical devices and pharmaceuticals, striving for the convenience, satisfaction, and trust that our users and patients rightfully deserve.
- Main Markets
-
 Australia
Australia
 China
China
 Germany
Germany
 India
India
 Poland
Poland
 Spain
Spain
 Turkey
Turkey
 U.S.A
U.S.A
- Main Product
Related Products

eyelash essence

Catch Me Patch Her's Band

LED Dark Circle Therapy

Hairview

Pentagon Grand (CO2 fractional laser)